modified fatigue impact scale
 Measurement Characteristics and Clinical Utility of the Modified Fatigue Impact Scale in Individuals With Multiple Sclerosis - Archives of Physical Medicine and Rehabilitation
Measurement Characteristics and Clinical Utility of the Modified Fatigue Impact Scale in Individuals With Multiple Sclerosis - Archives of Physical Medicine and RehabilitationWarning: The NCBI website requires JavaScript to operate. Psychometric properties of the scale of impact of modified fatigueSummary psychometric evaluations are tests or questionnaires that have been designed to measure the constructs of interest in an individual or objective population. A goal of many of these self-reporting instruments is to provide researchers with the ability to collect subjective information in a way that can allow quantitative analysis and interpretation of these results. This requires the instrument of choice to have appropriate psychometric properties of reliability and validity. Much research has been done on the creation of self-reported quality of life questionnaires for people with multiple sclerosis (MS). This article focuses on one in particular, the Modified Fatigue Impact Scale (MFIS). The item begins with a brief description of the rationality, construction and score of the inventory. The best reliability and validity data available in the MFIS are presented below. The article concludes with a brief discussion on the interpretation of the scores, followed by suggestions for future investigations. This summary analysis is intended to examine whether the instrument is adequately measuring the impact of fatigue and whether the scores allow for significant interpretations. Multiple sclerosis (MS) is a chronic, progressive and degenerative disease of the central nervous system characterized by demystification and axonal deterioration. Symptoms of the EM include abnormal gait, poor balance, muscle weakness, spasticity and fatigue, all of which can reduce physical function and deeply affect health and quality of life. Fatigue is one of the most common symptoms, affecting more than 75% of people with MS., Fatigue has been defined as "a subjective lack of physical or mental energy that is perceived by the individual or caregiver to interfere with daily life activities." Fatigue can be considered primary or secondary. Primary fatigue refers to factors directly associated with the disease process, while secondary fatigue relates to the consequences of primary fatigue and may result from lack of conditioning, depression and side effects of the medication. Despite the well-known prevalence of MS fatigue and its negative effects on quality of life, physiopathology of MS-related fatigue remains unclear. Furthermore, the difficulty of measuring fatigue due to its subjective nature and its impact on quality of life has been problematic. According to the Council of Multiple Sclerosis for Clinical Practices and Guidelines, a subjective (self-reported) measure of fatigue should be based on the individual assessment of fatigue and its impact on quality of life, which requires the measure to have appropriate psychometric properties, including reliability and validity. After a literature review, the Council recommended using the Modified Fatigue Impact Scale (MFIS). Although the Council called for continued psychometric evaluation, researchers have repeatedly used MFIS in the absence of such a comprehensive assessment of the instrument. The MFIS is a modified version of the Fatigue Impact Scale of 40 points (FIS), which was originally developed to evaluate the effects of fatigue on quality of life in patients with chronic diseases, specifically MS. The FIS has patients who evaluate the extent to which fatigue has affected their lives in the last four weeks in a questionnaire consisting of 10 "physical" articles, 10 "cognitives" and 20 "socials", with 0 indicating "no problem" and 4 indicating "a problem." The maximum possible score is 160. MFIS evolved from FIS during the development of a clinical inventory by evaluating the overall quality of life in individuals with MS, the Quality of Life of Multiple Sclerosis (MSQLI). During the field tests of MSQLI phase 2, the 40-point FIS was abbreviated at the 21-point MFIS for "eliminating elements that appeared both redundant in content and had high inter-themed correlations," but the exact procedures and data have not been published. The MFIS contains 9 "physical" articles, 10 "cognitive" articles and 2 "psychosocials". The maximum possible score is 84, with higher scores that indicate a greater impact on quality of life (). The original intention was to use the total score to reflect a global (unidimensional) score. The authors of the FIS and those involved in the modification of the FIS in the MFIS have not published evidence to verify the underlying structure of the instrument or the rationality of the three subscales and the elements selected to create subscales. The underlying structure of MFIS has been examined through analysis of main components with varimax rotation using data from 181 MS patients from four different European countries: Belgium (n = 51), Italy (n = 50), Slovenia (n = 50), and Spain (n = 30). All 21 items comply with the required item loading factor of 0.50. However, the first article, "I feel less alert" (a cognitive element), had a charged factor of 0.495 but also charged with the psychosocial factor to 0.599. The eighth theme, "I feel less motivated to participate in social activities" (a psychosocial item), had a charged factor of 0.58 but also charged with the physical factor to 0.499. The ninth theme, "I am less motivated to do things out of the house", of the psychosocial subscale, was actually assigned to the physical factor. These results do not agree with the original underlying structure of subscales, leading Kos and colleagues to recommend caution in interpreting psychosocial subscale. The lack of agreement in the underlying structure could confuse MFIS interpretations as a result. The total MFIS score ranges from 0 to 84. The ranges of scores for each subscale are as follows: physical, 0 to 36; cognitive, 0 to 40; and psychosocial, 0 to 8. To date, no data on population standards have been published for the Ministry of Finance and its subscales. Some studies use a total score of 38 as a cut to discriminate fatigued from non-fat individuals., The score of 38 was based on a study that correlated the MFIS with another fatigue inventory and its defined stitches for fatigue and without fat. The verification of these results has been less than adequate, and researchers simply cite the study of Flachenecker et al. as a justification for using a score of 38 to discriminate fatigued from non-fat individuals with MS. The lack of demographic standards and a possibly inappropriate court score raise some questions about the instrument and the ability to interpret scores. Reliability The reliability of MFIS has been evaluated in a small number of items, and in the MSQLI technical inventory, which includes MFIS. When considering reliability, two questions come to mind: 1) Is MFIS reliable (internal consistency)? 2) Is the impact of fatigue experienced by individuals with stable and reproducible MS with repeated measurements? The MSQLI technical inventory provides some of the best reliability tests (internal consistency). The field test of phase 2 of the MSQLI instrument studied a sample of 300 individuals with MS selected from 4 MS clinics in Canada. The sampling of topics focused on gender and a commonly used measure of disability, the Expanded Scale of the State of Disability (EDSS), keeping it similar to that of previous epidemiological studies. The reported internal consistency of all the scores of the MFIS was "excellent", with the following values of the α of Cronbach: total, 0.81; cognitive, 0.95; physical, 0.91; and psychosocial, 0.81. The technical guide suggests that MFIS can be used as a full (total point) and multidimensional (separate subscales) evaluation of fatigue impact. The internal consistency reported by Kos et al. in 2005 showed similar results for the total score and two of the subscales, physical and cognitive (Cronbach α values of 0.92, 0.88 and 0.92, respectively). However, the Cronbach α for the psychosocial subscale was 0.65. The lack of agreement with the psychosocial subscale could be explained by the fact that studies involved people with different cultural backgrounds, which could also influence other subscales. Lack of agreement does not invalidate subscale, but suggests that cultural differences as a confusing variable continue to be explored. The second question regarding reliability refers to whether the impact of fatigue experienced by individuals varies from one test to another. MFIS has been used as a result measure in several clinical trials, which means that the impact of fatigue persists and is the same within a person and through repeated measures. Some studies have been carried out to investigate the reproducibility of the MFIS test., In 2003, Kos et al. carried out a study in which the participants (n = 51) completed the MFIS on two separate occasions at the same time of the day. The subscales, together with the total score, were considered stable for 3 days using a test of Wilcoxon's range sum to evaluate the differences from day to day. Similar results were observed in a later study in 2005 in a much larger sample (n = 181). Once again, MFIS was administered on two occasions three days apart, without significant difference in scores (intraclass correlation ratio [CCI] of subscales ranged from 0.84 to 0.91 and the total was 0.91). However, a limitation of this study is the lack of explanation in the methodology regarding the administration of MFIS and whether confusing variables such as sleep or caffeine intake were accounted for. These data suggest that MFIS has proper test reliability; however, due to the possibility of confusing variables that affect the results of the tests, this reliability must be verified by performing a study in which these variables are controlled. Another confusing aspect that could affect reproducibility is the notion that fatigue may or may not be stable in a person with MS. Validity An instrument can be reliable but not valid; this is similar to the important distinction between precision and precision. The validity refers to how well the instrument is measuring the construction of interest. In assessing whether an instrument is valid, the nomological network under which the instrument was built should be considered. The nomological network under which MFIS was built has not been established or clarified directly. Therefore, we must first identify which factors should be very well correlated with MFIS, as well as those factors that must be unrelated or weakly correlated. If the construct is evaluating what it intends to evaluate, it must be correlated very well with other measures of similar constructions (convergent value), and it must be correlated weakly with constructions that are theoretically non-related (discriminatory value). One way to evaluate these validity aspects is to simultaneously manage MFIS with other related and non-related psychometric instruments and evaluate the strength of their correlations. Because MFIS evaluates the impact of fatigue, it must converge with other subjective measures of fatigue. The MFIS has moderate to strong correlations with the Fatigue Severity Scale (FSS), a general fatigue measure, which is not surprising since the FIS was derived from the FSS. Tellez et al. reported correlations between FSS scores and the total score of MFIS, with a correlation coefficient of 0.68. The subscales also correlate with the FSS, with correlation coefficients of 0.75 for the physical subscale, 0.44 for the cognitive subscale, and 0.62 for the psychosocial subscale. Kos et al. reported a 0.66 correlation between the FSS and the total score of MFIS. With both instruments that evaluate fatigue, a much greater correlation could be expected; however, MFIS should be considered primarily to assess the impact of fatigue, while FSS also evaluates severity and frequency. When the FSS is correlated with separate subscales of the MFIS, the physical subscale shows a stronger correlation than the other subscales. This is not surprising given that the impact of fatigue questions on the FSS focuses more on the physical aspects of daily life. The results of phase 2 evaluating the validity of MSQLI indicated significant correlations of the 36-point Health Status Survey (SF-36) vitality scale (r = −0.59), the disease impact profile (IPS) Sleep and rest scale (r = 0.47), and SIP Alert Scale (r = .65) with MFIS. Both SF-36 and IAPA are widely used instruments. The correlation with the scale of vitality helps to establish validity, because it is logical that fatigue can be accompanied by a decrease in vitality. The correlations with the Sleep and Rest Scale and the Alert Scale may suggest problems with the MFIS in which it cannot distinguish between sleepiness and alertness regarding the impact of fatigue. Depression could be a symptom of fatigue, and fatigue could be a symptom of depression, which would lead to a long-standing debate on the causal direction between these variables. Tellez et al. found a clear relationship between depression and fatigue in individuals with MS, with a significant correlation (r = 0.70) between the total score of MFIS and the measured depression with the Beck Depression Inventory (BDI). Depression and fatigue are often associated with specific variables of diseases such as disability and the duration of diseases. When EDSS scores were controlled, the correlation between depression and fatigue remained positive. When these variables were analyzed using multiple linear regression, EDSS score, depression and duration of the disease were all independent predictors of all subscales and total MFIS scores. However, Greim et al. reported that depressed MS patients were subjectively more tired than non-depressed MS patients (mean [SD] = 49.8 [13.1] vs. mean [SD] = 32.6 [16.8]; P The validity of a subjective instrument can be strengthened when combined with an objective measure, provided that the measures are appropriate substitutes. The study carried out by Greim et al. objective and subjective measures evaluated of mental and physical fatigue in individuals with and without MS. Participants completed the subjective fatigue measures, specifically MFIS, before and after a 30-minute surveillance test (an objective test of mental fatigue) and a manual dynamometer test (an objective test to evaluate physical fatigue). Depression was controlled during the study due to its possible influence on subjective and objective fatigue measures. A significant correlation was observed between subjective feelings (MFIS) and objective measures in individuals with MS. People with higher levels of subjective fatigue carried out worse objective measures. However, the scale of depression (BDI) was significantly correlated with fatigue measures, confirming previous data that indicate an interaction between fatigue and depression that can confuse the results of the study and subsequent interpretations. Considerable research has been undertaken to find ways to reduce the impact of fatigue on individuals with MS. Several pharmacological agents are used for this purpose, and data from these studies can provide evidence of validity for MFIS. Modafinil (Provigil), a commonly prescribed medication used to reduce symptoms of fatigue with the objective of reducing the impact of fatigue on quality of life, was tested during a 9-week trial of a single blind one involving a sample of 72 individuals with MS. All participants received the medication, but the treatment sequence was blinded. The drug regime included a placebo delivery period (weeks 1–2), 200 mg/day of fashionfinil (weeks 3–4), 400 mg/day of fashionfinil (weeks 5–6), and a placebo washing period (weeks 7–9). MFIS was one of the main results variables of this study. The total MFIS score, together with the scores for the three subscales, was significantly reduced when 200 mg/day of fashionable was administered. The actual average score went from 44.7 to 37.7 (7 reduction points). However, when participants were given the highest dose (400 mg/day), the average score of MFIS (42.1) does not differ from the time of placebo execution. The average score of MFIS after the placebo wash period was approximately the same as that after the placebo execution period (43.0 and 44.7, respectively). This provides some evidence that MFIS can detect a change in scores; however, the lack of a true control group and the lack of control for other variables such as sleep and depression can confuse the results. These results suggest that MFIS is sensitive to change, but that the interpretation of these results is limited to that: a reduction in the scores of MFIS. Little to no research has been published indicating what change in the scores of MFIS (increase or decrease) reflects objectively. Currently, interpretation is limited to changing directions, i.e. a reduction in scores, no significant change in scores or an increase in scores. The term clinically significant or clinically relevant has been used in several studies in an attempt to give meaning to the results and allow some sort of interpretation of the scores. However, these studies do not provide sufficient evidence for their selection of what is considered clinically significant. A study by Kos et al. considered a change in the score of 10 or more to be clinically relevant, based on other studies that found a difference in the scores of MFIS from 7 to 20.1. Another article used a cutting point of 45 or more for the total score of MFIS as a study input criterion, without providing a clear justification for the selection of that score. The lack of objective anchors for the total and subscale of MFIS limits the interpretations of the results of this instrument. A more recent article found flaws in the underlying structure of MFIS and used the Rasch measurement model to determine that the use of the total score is invalid. The Rasch model represents the interactions between subjects and the test elements to produce linear measurements: "The model affirms that the probability of a person to give a given answer to an article is a logistical function of the difference between the 'capacity' of the person and the 'dificulty' of the article." In the case of MFIS, a person with high levels of fatigue would assert articles that express high levels of fatigue, while a person with low levels of fatigue would have difficulty asserting these articles. According to Wright and Linacre, it is the only way that ordinary observations of clinical phenomena can be converted into linear measurements. The 21-point MFIS did not fit in the Rasch model, which does not support the use of the total score as a global index, but indicates that it is multidimensional. Mills and colleagues further explored this concept and were able to achieve fit to the Rasch model through the removal of the following items: 4 ("I'm awkward and uncoordinated"; physical subscale), 14 ("I'm physically uncomfortable"; physical subscale), and 17 ("I'm less capable of completing tasks that require physical effort; physical subscale). The authors then identified two subscales of the MFIS: physical and cognitive. The psychosocial subscale was eliminated because the articles were found as part of the physical subscale, consistent with the findings of Kos et al. The authors argue that studies in which the overall score of MFIS was used as a result measure or a selection tool could be invalid or subject to misinterpretations. Despite problems related to the underlying structure, it seems that physical and cognitive subscales can be useful as results measures. Conclusion Clearly, MFIS as a result measure has some problems that result in limitations to interpret scores. The lack of agreement between the underlying structure and its subscales poses complex problems, especially if the total MFIS score is null. The total MFIS score has been commonly reported as a result measure, but given the results reported by Mills et al., the interpretations of the studies using the total MFIS scores may need to be re-evaluated. In addition, the potential effects of confused variables—specifically, depression—in MFIS scores can lead to a poor interpretation of uncontrolled study results. There may not be a good way to separate these constructs in some samples as people with MS. Probably the biggest problem with MFIS score interpretation is the lack of objective anchors. As the measurement is located, interpretations are limited to whether a score changed significantly and the direction of that change. Studies have not yet been published that objectively show what represents a change in the MFIS. In other words, if the score of physical subscale of a MS patient on MFIS improves at 5 points, does that mean that the person can walk further and faster? More research on MFIS is needed to address some of the problems described here and to identify what change in the MFIS score represents to an objective measure of quality of life in the physical and cognitive domains. PracticePoints The Modified Fatigue Impact Scale (MFIS) is a widely used self-reporting measure of fatigue in people with MS, but its reliability and validity have not been adequately addressed. MFIS has several problems that result in limitations to interpret scores. In particular, the lack of agreement on the scale between the underlying structure and its subscales poses complex problems and should be considered before using this instrument in clinical and practical research. Appendix 1. Financial Modified Fatigue Impact Scale Disclosure: The author has no conflict of interest to reveal. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA

Fatigue classification. MFIS, Modified Fatigue Impact Scale; MS,... | Download Scientific Diagram
STOP, THAT and One Hundred Other Sleep Scales
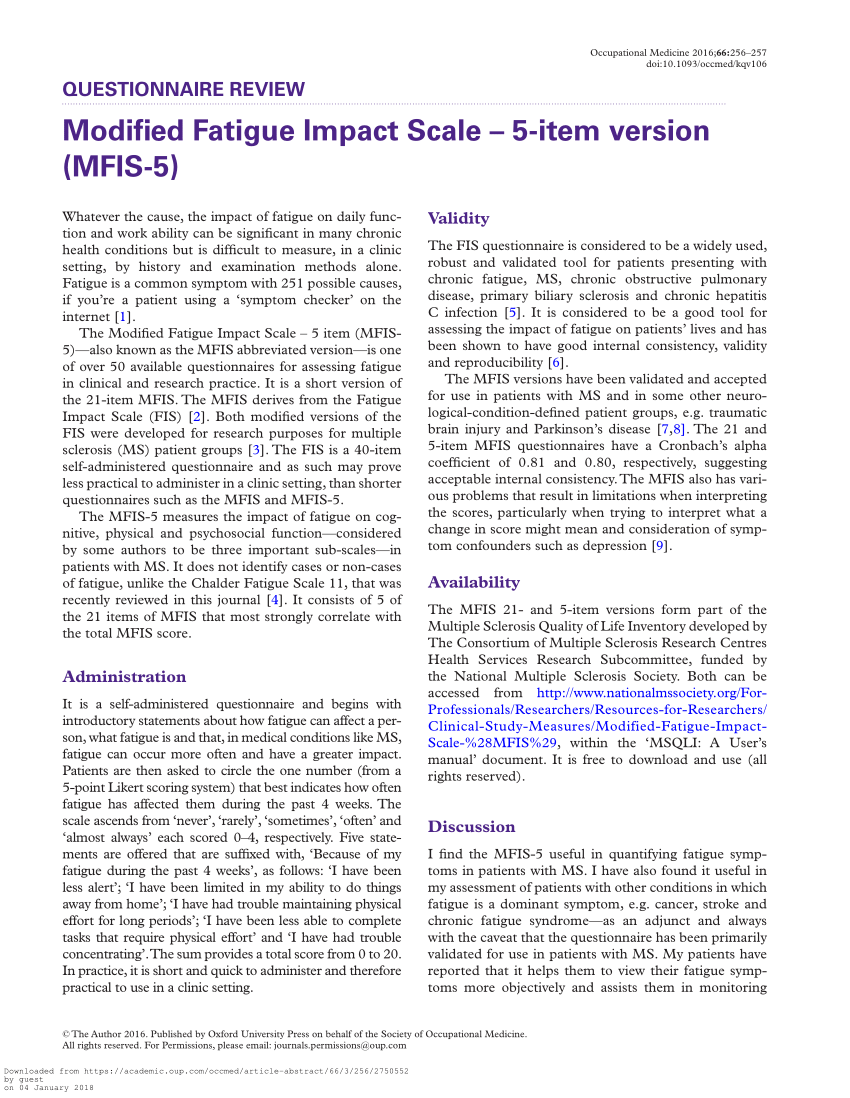
PDF) Modified Fatigue Impact Scale - 5-item version (MFIS-5)
![PDF] Validation of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar PDF] Validation of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b433483c1621025294a6659b9fc59124eda8a2e8/3-Table2-1.png)
PDF] Validation of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar

Minimal clinically important difference of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar

PDF) Evaluation of the Modified Fatigue Impact Scale in four different European countries
![PDF] Validation of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar PDF] Validation of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b433483c1621025294a6659b9fc59124eda8a2e8/3-Table3-1.png)
PDF] Validation of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar

MFIS - Modified Fatigue Impact Scale

PDF) Evaluation of the Modified Fatigue Impact Scale in four different European countries
MFIS - "Modified Fatigue Impact Scale" by AcronymsAndSlang.com

Figure 7, Change From Baseline in Modified Fatigue Impact Scale - Clinical Review Report: Ocrelizumab (Ocrevus) - NCBI Bookshelf
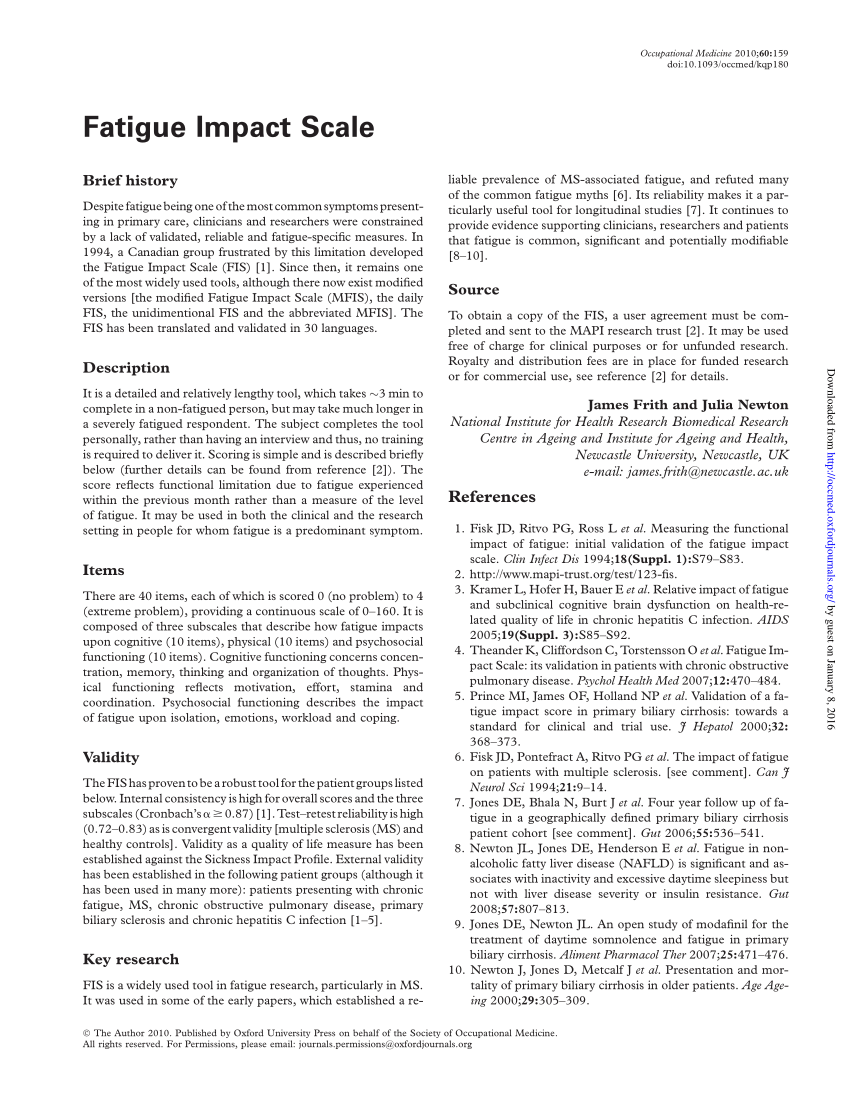
PDF) Fatigue Impact Scale
Original articles Assessing fatigue in multiple sclerosis : Dutch Modified Fatigue Impact Scale

Modified Fatigue Impact Scale Questionnaire | Semantic Scholar
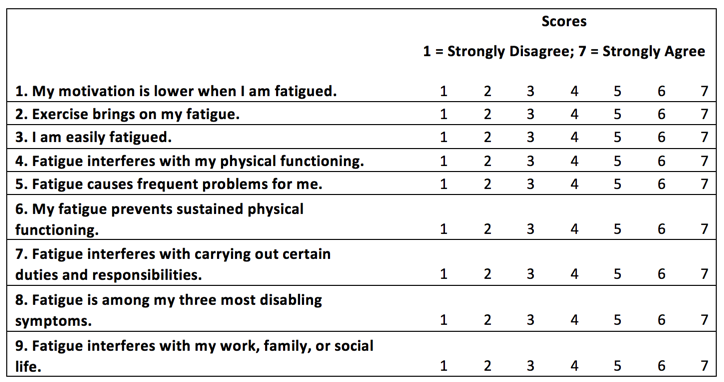
Self-Report Questionnaires: Fatigue and Sexual Function - Multiple Sclerosis Centers of Excellence

Fillable Online Modified Fatigue Impact Scale 5-Item Version - MedStar Health Fax Email Print - pdfFiller
Mfis Scale Ms - Drone Fest
Measurement properties of a telephone version of the Modified Fatigue Impact Scale among individuals with a traumatic spinal cor
STOP, THAT and One Hundred Other Sleep Scales

Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis - ScienceDirect

View Image

An external file that holds a picture, illustration, etc. Object name is i1537-2073-15-1-15-t01.jpg | Medical conditions, Chronic fatigue, Self
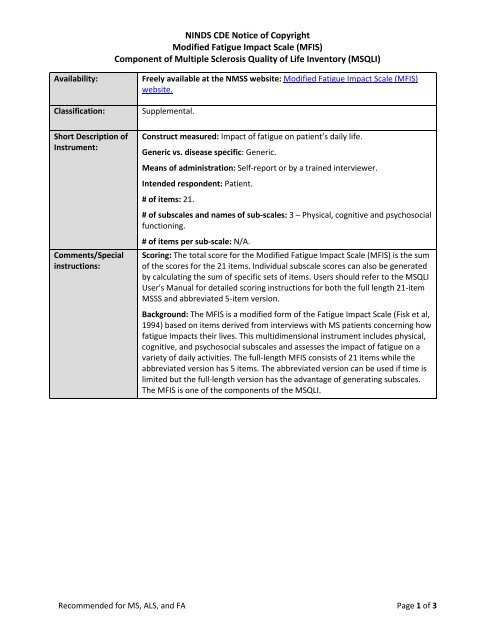
Modified Fatigue Impact Scale - NINDS Common Data Elements

FRI0583 VALIDATION OF THE MODIFIED FATIGUE IMPACT SCALE IN DANISH PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS | Annals of the Rheumatic Diseases
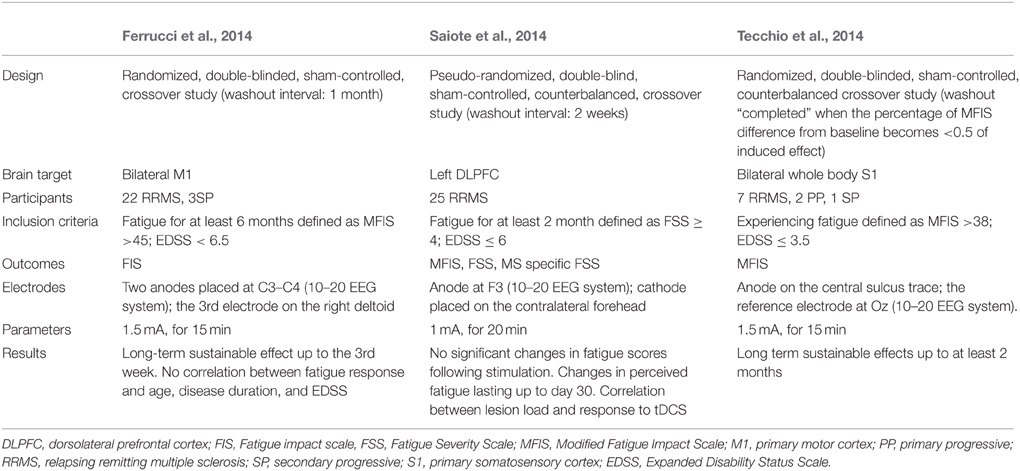
Frontiers | Fatigue in Multiple Sclerosis: Neural Correlates and the Role of Non-Invasive Brain Stimulation | Cellular Neuroscience
![PDF] Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? | Semantic Scholar PDF] Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8515c142bca2922f363da0c14f30ac9ae39075c5/4-Table2-1.png)
PDF] Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? | Semantic Scholar

US20070225368A1 - Therapy for Multiple Sclerosis - Google Patents

Top PDF Modified Fatigue Impact Scale - 1Library
PLOS ONE: Efficacy of Modafinil on Fatigue and Excessive Daytime Sleepiness Associated with Neurological Disorders: A Systematic Review and Meta-Analysis

PDF) Assessing fatigue in multiple sclerosis: Dutch Modified Fatigue Impact Scale

Minimal clinically important difference of the Modified Fatigue Impact Scale in Parkinson's disease. | Semantic Scholar
Outline
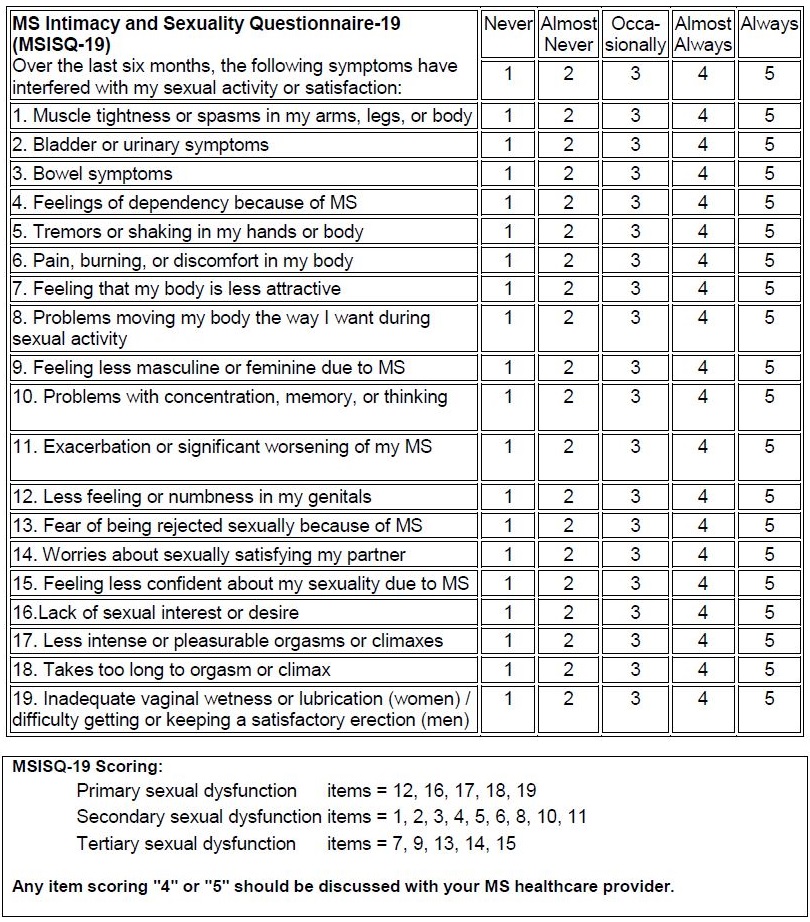
Self-Report Questionnaires: Fatigue and Sexual Function - Multiple Sclerosis Centers of Excellence
PDF) Psychometric Properties of the Modified Fatigue Impact Scale
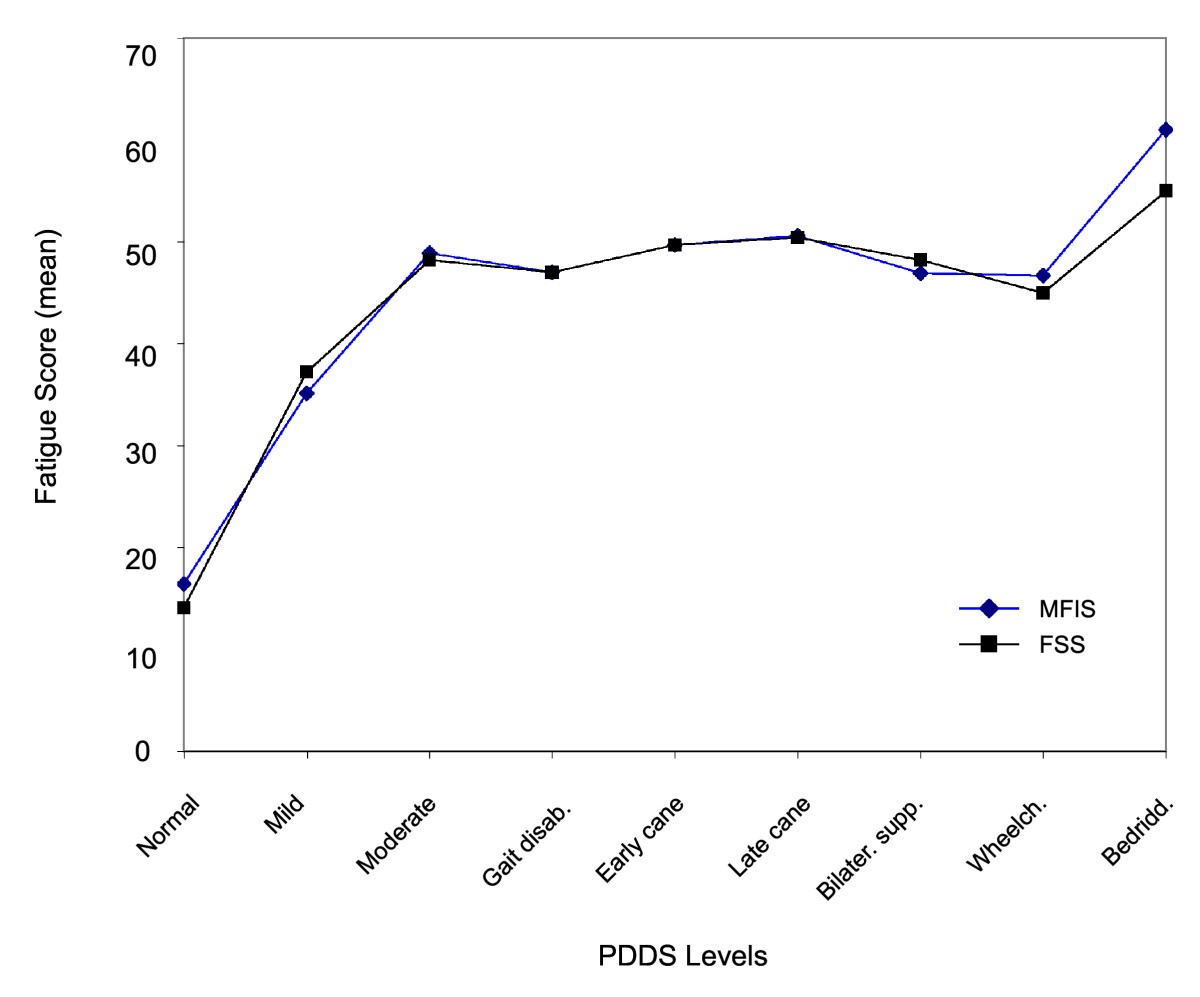
Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey | Health and Quality of Life Outcomes | Full Text
MFIS - Modified Fatigue Impact Scale

PDF) Assessing fatigue in multiple sclerosis: Dutch modified fatigue impact scale | Daphne Kos - Academia.edu

FRI0583 VALIDATION OF THE MODIFIED FATIGUE IMPACT SCALE IN DANISH PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS | Annals of the Rheumatic Diseases
Fatigue Severity Scale | RehabMeasures Database
Posting Komentar untuk "modified fatigue impact scale"