fasciculations of the tongue
 Tongue Fasciculations in Amyotrophic Lateral Sclerosis | NEJM
Tongue Fasciculations in Amyotrophic Lateral Sclerosis | NEJMPublicity Language Fasculations with Density Pattern in Osmotic Demisilation Syndrome: a Case Report Diagnostic Dilemma volume 11, article number: 177 (2018) volume 112658 AccessesAbstractBackground Patogenesis of osmotic demysylation syndrome is not fully understood and usually occurs with severe and prolonged hyponatremia, particularly with rapid correction. It can occur even in North-monathremic patients, especially with risk factors such as alcoholism, malnutrition and liver disease. Bilateral language fasciculations with electromyogram denervation pattern is a manifestation of damage to the hypoglossal nucleus or hypoglossal nerves. Language fasciculations were rarely reported in some cases of osmotic demylation syndrome, but the exact mechanism is not explained. Case presentationA 32-year-old Sri Lankan man, with a daily alcohol and alcohol consumption history and binge drink, presented with progressive difficulty walking, dysphagia, dysarthria and drooling of saliva and altering consciousness. When examining it was an akinetic and rigid Parkinsonism with a positive sign of Babinski. The clinical characteristics were the diagnosis of osmotic demitalization syndrome and MRI showed an abnormal signal intensity within the central ponies and basal ganglia. He also had tongue fasciculations. The electromyogram showed denervation pattern in the tongue with normal findings in the extremities. Bilateral hypoglosal nerves and medulla were normal in magnetic resonance. Conclusion We were unable to explain the exact mechanism for the denervation of the language, which gave rise to the fasciculations in this chronic alcoholic patient who developed the osmotic demystification syndrome. Hypoglosal nuclei are found in the dorsal medulla and radiologically undetected myelinolysis of medulla is a possibility. Hypoglosal nerve damage caused by methanel or other toxic substances that may contaminate regular ethyl alcohol is another possibility, as it is known to cause neurological and radiological characteristics similar to osmotic demystification syndrome with long-term exposure. So these toxic substances could play a role in chronic alcoholic patients with central pontino myelinolysis. BackgroundDemylinization syndrome (ODS) or central pontin myelinolysis (PMC) is a severe and prolonged hyponatremia complication, especially with rapid correction. It results in non-inflammatory demystination within the central ponies and also in extra-pontine regions, including the middle brain, talamo, basal nuclei and cerebellum. ODS pathogenesis is not fully understood. During chronic hyponatremia, osmotically active substances and water are lost from brain cells and these solutes cannot be replaced quickly by the rapid correction of hyponatremia. These rapid changes in intracellular, extracellular, intravascular, sodium, chloride and organic osmolytes produce relative glial dehydration and myelin degradation and/or oligodendroglial apoptosis [, ]. Gankam Kengne et al. hypothesized that the rapid correction of hyponatremia triggers apoptosis of astrocytes, disruption of astrocyte-oligodendrocyte network, secondary inflammation with regulation of inflammatory cytokine genes, microglial activation that eventually leads to demystation []. Although it usually occurs after a rapid hyponatremia correction, it may occur even in North-monathremic patients, especially who have risk factors such as alcoholism, malnutrition, liver disease, liver transplant, diabetes mellitus, hypokalemia, pituitary surgery, lithium toxicity, hypophosphatemia, chemotherapy, and chronic kidney failure [,,,,,,,,]. The clinical manifestations of ODS include several degrees of encephalopathy, behavioral disturbances, lethargy, confusion, disorientation, obtundation, dysarthria and dysphagia [, ]. Patients severely affected may develop coma or be locked in syndrome [, ]. The involvement of the corticopine and corticobulin pathways within the ponies leads to the pseudo-bulbar palsy and the spastic quadrilegia. Extra-pontine myelinolysis leads to extra-pyramidal signs and Parkinson as manifestations [,]. The hypoglossal nerve is the 12th cranial nerve, which internalizes the muscles of the tongue. The damage to the hypoglossal nucleus or hypoglossal nerve (the lower motor neuron) results in weakness, atrophy and phosciculation of the tongue, which are signs of a lower motor neuron lesion. Bilateral-language fasciculations with electromyogram denervation pattern (EMG) are typically observed in the previous horn cell disease [], but are rarely reported in cases of damage to bilateral hypoglosal nerves along their path [,,,,,,,,]. As mentioned above, the SDG/WHC causes palliative pseudo-bulbar with a stomatic tongue. Here we describe a young man with a history of chronic alcoholism, who presented characteristics of ODS. Although their sodium was normal, the clinical manifestation and magnetic resonance findings (MRI) were typical of the AD. During recovery, it developed bilateral language fasciculations and MRI did not show any damage to the medulla or along the hypoglossal pathway. The language fasciculations were reported in some cases of FMCT rarely, but the exact mechanism was not explained []. Case presentationA 32-year-old Sri Lankan man, who lived in South Korea for the last four years admitted to us with an altered level of consciousness, who progressed for 2 weeks. While in Korea, he was drinking alcohol daily (Soju: "The most popular alcoholic beverage in Korea" 150 mL/day) and during weekends he used to tie the drink. After a booze he developed anorexia, nausea, lethargy, progressive difficulty walking, dysphagia, dysarthria and saliva bib, which was progressive. He then returned to Sri Lanka where his relatives noticed behavioral changes, reduced speech and progressive alteration of consciousness. There was no previous history of headache, fever, exposure to toxin and had no diabetes, hypertension, hypothyroidism, chronic liver cell disease, caffeine consumption, tobacco or illegal drugs. Their nutritional status was adequate and there was no family history of neurological disorders. When he was examined, he was a medium-built person who was sleepy, disoriented, akinetic and rigid like Parkinsonism. He had reduced speech and saliva bib. It had no ophthalmoplegia and the vision, background examination and light reflections were normal. The rest of the cranial nervous examination was also normal without any subtle cranium-bulbar atrophy. The upper and lower extremities were rigid with increased reflexes and positive Babinski sign. The power was difficult to assess due to stiffness. His sensory examination was normal and did not have muscle atrophy or fasciculations. He was afebrile and there were no signs of meningism or chronic liver cell disease. His basic research was normal including serum sodium, liver function tests and inflammatory markers (Table ). The blood box showed normal red blood cells and macrocytic and normal neutrophils. Serum B12 was normal. The electroencephalography showed diffuse bi-hemispheric deceleration. MRI, made 2 weeks after the symptoms appeared, showed an abnormal signal intensity within the central ponies with low signal intensity in T1 and high signal intensity in T2 and fluid-energyed investment recovery (FLAIR) (Fig. ). There was no improvement in contrast or restriction of diffusion. The pre-pontine cistern seemed normal without evidence of massive effects. Bilateral basal nodes also showed symmetrical hypertensity of T2 and FLAIR (Fig. ). These were suggestive of CPM with extra pontin myelinolysis involving basal ganglia. The analysis of the brainspinal fluid (CSF) was normal without cells, normal proteins and sugar levels. CSF was also negative for the reaction of the chain of polymerase tuberculosis, the culture of tuberculosis and oligoclon bands. Table 1 Fig. 1MRI, done 2 weeks after symptoms appear, showing high signal intensity within the central ponies and bilateral basal ganglia in fluid-stricken investment recovery Due to the extrapyramidal signs that started in syndopa (37.5 mg three times a day) and gradually recovered. The level of consciousness, speech and action became normal within 1 week. The minimum mental score test performed at this stage was normal (30/30). During follow-up, 2 weeks later, bilateral language fasciculations were observed without obvious waste during this time. (Additional File: Film 1) The rest of the cranial nerves were normal. Although the reflections of the upper extremity and the lower extremity were exaggerated, there was no waste, particles or weakness. He did not have papilledema, nistagmo of lattice or mirror of movements that suggest an injury to the level of magnum foramen. Their sensory examination of the system and the cerebellar test were normal. EMG showed changes in denervation (fibrillations, large amplitude, long duration, potential motor unit action complexes and a reduced interference pattern) in the genoglossal muscle suggesting a hypoglosial nervous injury of the lower motor type. EMG and nervous driving (NCS) studies of the limbs were normal. The magnetic resonance was repeated 1 month after the first magnetic resonance and showed the same changes (low T1 and high-intensity T2/FLAIR without restriction of diffusion or improvement of contrast) in the central pons (Fig. ), but no previously observed changes were seen in the basal nodes. At the same time we did a high-resolution magnetic resonance of the brain trunk and in this, abnormal signal intensities were located to the pons (Fig. ) not extending to the medulla (Figs. , ) and the bilateral hypoglosal and hypoglosal nerves were normal. Fig. 2MRI, made 1 month after the first MRI, showing high intensity FLAIR in the central pons with resolution of changes in the basal nodeFig. 3MRI of the brain trunk, made 1 month after the first MRI showing signal intensity in FLAIR located to the ponsFig. 4MRI of the brain trunk, made 1 month after the first MRI that shows normal menuclela and hypog. 5MRI of the brain trunk, made 1 month after the first coronal views of the MRI, showing high signal intensity located to the ponies and not extending to the medulla His tests of thyroid function, fasting of blood sugar, HbA1c, lipid profile, chest X-ray and ultrasound of the abdomen were normal. Human immunodeficiency virus 1 and two antibodies, the Venerous Disease Research Laboratory, the superficial antigen of hepatitis B, hepatitis C antibody and antinuclear antinuclear antibody were negative. The level of serum ceruloplasm was normal and the examination of the lamp did not reveal Kayser-Fleischer rings. We were unable to organize a CT scan of the total body to look for hidden malignities that can be lost in chest X-ray and ultrasound abdomen and during follow-up there was no symptoms or signs of such. Discussion and conclusion Our patient had typical ODS characteristics as a reduced discourse, dysarthria, dysphagia, behavioral disturbances, lethargy, confusion and disorientation. Hyperreflexia is a typical feature of the involvement of superior motor neurons or corticopine pathways within the pons. Parkinson as features can be explained by the osmotic extra pontin demythal of the basal nodes []. The current standard method for diagnosing osmotic demylation syndrome (ODS) is brain MRI and is very sensitive to detect myelinolysis []. The Pope's demystification remains the hallmark of the disease []. Our patient had the typical findings of MR [, ] of ODS and therefore the clinical and radiological evidence was compatible with our ODS diagnosis. Although our patient did not have a documented hyponatremia, it is not a need in the ODS/WHC [, , , , , ,,,,,,,]. In a systematic review of studies on central pontin and extrapontin myelinolysis around a quarter of patients with ODS/WHC had sodium of more than 135 mmol/L []. Although our patient's sodium was normal, he could have had sodium low in the past with the ingestion of large quantities of beer called "beer potomania". In this patient the management was mainly symptomatic and we started the syndopa for extrapyramid symptoms. Insulated cranial nerve paralysis is rare at CPM. The sixth left-wing nervous paralysis was reported in a 30-year-old PRIME with PPM and in this case the lesion of the MRI anatomically correlated to the location of the sixth nervous fascicle []. The most interesting clinical manifestation in our patient is the fasciculation of the tongue with denervation pattern in EMG. To date, only some cases of this demonstration have been reported. A report describes a 46-year-old man with a history of alcohol abuse who presented alcohol withdrawal and developed MPC. He had tongue fasciculations with a deviation from the tongue to the left in protrusion []. In this case it was postponed that the presence of fasciculations and deviation of languages suggests dysfunction at the level of the hypoglossal motor core in the medulla or more peripherally justifying a greater work. The hypoglosal nuclei are located in the median eminence of the dorsal medulla near the dorsal nerve core Vagus and the solitarius tract nucleus. In our patient the MRI (1.5 Tesla) showed no lesion in the medulla that could involve hypoglossal nucleus. However, radiologically undetected myelinolysis is possible in bilateral medulla and hypoglosal nerves, which could have been seen with a stronger force magnet. The fibers that arise from the hypoglossal nuclei travel laterally to the median and medial lemnisco to the lower olive nucleus, run in the sulcus between the pyramids and the olives, then enter the hypoglossal channel and emerge extracranally. It is medial and post-carotid artery inside the carotid pod and crosses the cross-sectional process of the first vertebral body. Then it is executed earlier and internalizes the muscles of the tongue. The damage to both hypoglosal nerves will produce fasciculations and atrophy of tongue due to denervation. This was reported in cases with cerebral stem metastases [, ], post-traumatic condile fracture [, ], traction of nerves in vertical subluxation of the odontoid process [], in head injury [, ], after the use of the larynx mask via air [, ] and post carotidic endoctrectomy [, ]. In our patient we could not find any lesions that could damage the hypoglossal nerve in the RRM. Tongue fasciculations are also seen in benign fasciculation syndrome, amiotrophic lateral sclerosis, poliomyelitis, progressive bulbar pasy, spinal muscle atrophy, paraneoplastic syndromes. Acetylcholinesterase inhibitors, benzodiazepine abstinence, stimulants, organophosphate poisoning, magnesium deficiency and myasthenia gravis [], but these were unlikely in our patient. Long-term exposure to methanel, toluene, carbon and n-hexane can lead to damage to the central nervous system, pyramidal signs, parkinsonism and fasciculations [, ]. In a patient NCS and EMG had shown low-suggestioning fasciculations and waves of polyneuropathy []. A case report described a 34-year-old floor layer that developed motor neuron disease with fasciculations and EMG evidence, after being accidentally exposed to a mixture of solvents containing methanel and other substances []. In a survivor after methanel poisoning, an extensive denervation was observed in suggestive electromyography of the previous intervention of the horn cell []. Gille et al. described a patient with chronic motor neuropathy that resembles a pseudo-polyeuretic type of ALS as a sequel to methanel poisoning []. Bourrat et al. reported to a woman after voluntary methanol poisoning that had electromiographic signs of neurogenic atrophy []. These reports suggest that methanel may affect lower motor neurons. Like ODS, in methanel poisoning also MRI will show the involvement of basal ganglia, especially slutmen and caudate, and tegmentum pontino [,]. In such cases, the bilateral adjoining lesions appeared hyperintensate in weighted images T2 and hipointensas in weighted images T1 []. Methanol poisoning can occur with illegal distillation or concealing substitution for ethanol, which is possible in our patient. Most patients who presented with ODS with normal sodium levels were alcoholic as this case [, , , ]. Therefore, methanel and other toxic substances in alcohol could have played a role in these presentations as clinical manifestations and MRI findings of these poisoning can imitate ODS. Mast et al. It was also pointed out that restoration of iatrogenic sodium may not be the putative mechanism in all cases of PFC [] so that another pathology can coexist. In our patient we were unable to make the tox screen and the levels of methanel in admission due to financial difficulties. But it had no evidence of the nerve-optic involvement in the MRI or visual evoke potentials, which is seen in methanel poisoning. In conclusion, tongue fasciculations with nerve loss of the tongue muscles are rarely reported in patients with ODS/WHC. We could not explain the exact mechanism for this, but a possibility is radiologically undetected myelinolysis of medulla. Methanol or other toxic substances that can contaminate alcohol by damaging the hypogloss nerve and causing a lower motor type denervation is the other possible mechanism. Absorotic demining syndrome Central mielinolisis pontinaelectromyogrammagnetic resonance imagingfluid-atenuated inversion recoverycerebrospinal fluidnerve conduction studiesReferencesKumar S, et al. Central pontine mielinolisis, an update. Neurol Res. 2006;28(3):360-6. Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolites during the correction of chronic hyponatremia. Implications for the patogenesis of the central pontin myelinolysis. J Clin Invest. 1991;88(1):303–9. Gankam Kengne F, et al. Astrocytes are an early goal in osmotic demitalization syndrome. J Am Soc Nephrol. 2011;22(10):1834–45. Jha AA, et al. Osmotic demysylation syndrome in a North-monatromic patient with chronic kidney disease. Indian J Crit Care Med. 2014;18(9):609-11. Mascalchi M, Cincotta M, Piazzini M. Case report: MRI demonstration of pontin and thalamic myelinolysis in a Northmonatremic alcoholic. Clin Radiol. 1993;47(2):137-8. Lohr JW. Osmotic demystification syndrome after hyponatremia correction: association with hypokalemia. Am J Med. 1994;96(5):408–13. Koul PA, et al. Osmotic demystification syndrome after slow hyponatremia correction: possible role of hypokalemia. Indian J Crit Care Med. 2013;17(4):231-3. Mast H, et al. Mielinolisis central pontin: clinical syndrome with normal serum sodium. Eur J Med Res. 1995;1(3):168–70. Mohammed AS, Boddu P, Yazdani DF. Clinical evolution of central myelinolysis of pontin in a patient with alcohol withdrawal: a blurry clinical horizon. Case Rep Med. 2016;2016:6065259. Singh TD, Fugate JE, Rabinstein AA. Central and extrapontine pontine mielinolisis: a systematic review. Eur J Neurol. 2014;21(12):1443–50. Karp IB, Laureno R. Pontine and extrapontin myelinolysis: a neurological disorder after rapid hyponatremia correction. Medicine. 1993;72(6):359–73. Sterns RH, Riggs JE, Schochet SS Jr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314(24):1535–42. Sixer A, et al. Parkinsonism and dystonia in central pontine and extrapontine myelinolysis. Neurosurgery Psychiatry J. 1998;65(1):119–21. Kwon DY, et al. Parkinsonism and rapid deterioration dysautonomia in patients with central myelinolysis pontin and extrapontin. Neurosurg Clin Neurol. 2011;113(6):513-4. Sullivan AA, Chervin RD, Albin RL. Parkinsonism after hyponatremia correction with central myelinolysis radiological pontin and changes in the basal nodes. J Clin Neurosci. 2000;7(3):256-9. Wijesekera LC, Leigh PN. Amiotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. Bageant TE, Tondini D, Lysons D. Bilateral hypoglossal-nerve paramesis after a second carotide endoretomy. Anesthesiology. 1975;43(5):595-6. Brennan RJ, Shirley JP, Compton JS. Bilateral hypogloss nerve paralysis after head injury. J Emerg Med. 1993;11(2):167-8. Keane JR. Atrophy of the tongue of cerebral trunk metastases. Arch Neurol. 1984;41(11):1219–20. Kenrick MM, et al. Bilateral injury to the hypogloss nerve. Arch Phys Med Rehabil. 1977;58(12):578–82. Rotta FT, Romano JG. Base cramps metastasis that cause acute bilateral hypogloss nerve palliative. J Neurol Sci. 1997;148(1):127-9. Shahzadi S, Abouzari M, Rashidi A. Traumatic bilateral traumatic hypogloss nerve transsection in an explosion injury. Surg Neurol. 2007;68(4):464-5. Sommer M, et al. Bilateral hypogloss nerve injury following the use of the laryngeal mask without the use of nitrous oxide. Acta Anaesthesiol Scand. 2004;48(3):377-8. Bakst R, Kasper M, Greene R. Central pontine myelinolysis in a patient admitted for alcohol withdrawal. Hosp Physician. 2008;44:23-8. de Souza A. Akinetic-rigid syndrome due to extrapontin and pontin myelinolysis after the proper correction of hyponatraemia. J Clin Neurosci. 2011;18(4):587-9. Rippe DJ, et al. Images of central myelinolysis of pontin. J Comput Assist Tomogr. 1987;11(4):724-6. Kilinc M, Benli US, Can U. Osmotic myelinolysis in a normonatremic patient. Acta Neurol Belg. 2002;102(2):87-9. Carrington JM, Sánchez G, Berkeley JL. Central pontine myelinolysis with minimal hyponatremia in the setting of AIDS. Case Rep Neurol Med. 2015;2015:421923. Yamashita C, et al. A case of central pontin myelinolysis caused by hypophosphatemia secondary to refeeding syndrome. Case Rep Neurol. 2015;7(3):196–203. Saini M, Mamauag MJ, Singh R. Central pontine myelinolysis: a rare secondary presentation to hyperglycemia. Singapore Med J. 2015;56(4):e71-3. Burns JD, Kosa SC, Wijdicks EF. Mielinolisis central pontin in a patient with hyperosmolar hyperglycemia and normal serum sodium. Neurocritical attention. 2009;11(2):251-4. McNamara PH, et al. Central pontin mielinolysis in a patient with alcohol dependency syndrome without hyponatremia. JAMA Neurol. 2016;73(2):234-5. Fenves AZ, Thomas S, Knochel JP. Poomania beer: two cases and literature review. Clin Nephrol. 1996;45(1):61-4. Gosavi TD, See SJ. Central pontine mielinolisis that presents as a sixth nervous paralysis isolated in the third trimester of pregnancy. Ann Indian Acad Neurol. 2015;18(1):84-6. Orbay T, et al. Late hypogloss nerve paralysis after fracture of the occipital condylium. Surg Neurol. 1989;31(5):402-4. Lam CH, Stratford J. Bilateral hypoglosal nerve injuries with occipital condilar fracture. You can J Neurol Sci. 1996;23(2):145-8. Macedo TF, et al. Bilateral hypogloss nervous disorder due to vertical subluxation of the odontoid process in rheumatoid arthritis. Br J Rheumatol. 1988;27(4):317–20. Stewart A, Lindsay WA. Bilateral hypogloss nerve disease following the use of the laryngeal mask airway. Anaesthesia. 2002;57(3):264-5. Gutrecht JA, Jones HR Jr. Bilateral hypogloss nerve injury after bilateral carotid endoretomy. Stroke. 1988;19(2):261–2. Burch J, et al. Miastenia gravis – a rare presentation with atrophy and fasciculation of the tongue. Age. 2006;35(1):87-8. Hageman G, et al. Parkinsonism, pyramidal signs, polyneuropathy and cognitive decline after exposure to long-term occupational solvents. J Neurol. 1999;246(3):198–206. McLean DR, Jacobs H, Mielke BW. Methanol poisoning: clinical and pathological study. Ann Neurol. 1980;8(2):161-7. Chio A, et al. Motor neuron disease and optical neuropathy after acute exposure to a solvent mixture containing methanel. Amyotroph Lateral Scler Other Disord of Neuron Motor. 2004;5(3):188–91. Gille M, et al. Motor neuropathy, whore necrosis and optic nerve atrophy after acute methanel poisoning. Rev Neurol. 1998;154(12):862-5. Bourrat C, et al. Voluntary methanol poisoning. Severe regressive encephalopathy with anomalies in computed x-ray tomography. Rev Neurol. 1986;142(5):530-4. Gaul HP, et al. MR findings in methanol poisoning. AJNR Am J Neuroradiol. 1995;16(9):1783–6. Rubinstein D, Escott E, Kelly JP. Methanol poisoning with white and whoreminal necrosis: MR and TC. AJNR Am J Neuroradiol. 1995;16(7):1492-4. Arora V, et al. MRI findings methanol poisoning: a two-case report. Br J Radiol. 2007;80(958):e243-6. Contributions from authors HMMTBH collected data, followed the patient and performed the literature review and wrote the manuscript. SPP and SS helped collect data, follow the patient and correct the manuscript. All authors read and approved the final manuscript. Recognition This report was supported by doctors working in room 16, Sri Lanka's national hospital, in the acquisition, analysis and interpretation of data. We are grateful to the relatives of patients for the support provided in the provision of data. Competing interests The authors declare that they have no competing interests. Availability of data and materials Data sets that support the conclusions of this article are included in the article. Consent to publicationThe written informed consent was obtained from the patient for the publication of this case report and any accompanying image. Ethics and consent to participate Not applicable. Funding No funding source. Editor's Note Nature remains neutral with respect to jurisdictional claims on published maps and institutional affiliations. Author's information National Hospital, Colombo, Sri Lanka H. M. T. B. Herath, S. P. Pahalagamage " Sunethra Senanayake You can also search this author in You can also search this author in You can also search this author in Correspondence to the correspondent .Additional File 1: Movie 1. Bilateral language Fasculations. Data description: Bilateral language fasciculations were observed without obvious waste during follow-up. Rights and permits Open access This item is distributed under the terms of the Creative Commons 4.0 International License (), which allows the use, distribution and reproduction without restrictions in any medium, provided that you give appropriate credit to the original author(s) and source, provide a link to the Creative Commons license, and indicate whether changes were made. The Creative Commons Public Domain Dedication () exemption applies to the data available in this article, unless otherwise stated. Open access About this article Cite this article Herath, H.M.M.M.T.B., Pahalagamage, S.P. " Senanayake, S. Tongue fasciculations with denervation pattern in osmotic demyelination syndrome: a case report of diagnostic Dilemma. BMC Res Notes 11, 177 (2018). https://doi.org/10.1186/s13104-018-3287-811, Received: 18 September 2017Accepted: 07 March 2018 Published: 14 March 2018DOI: Keywords BMC Research Notes ISSN: 1756-0500 Contact us BMC By using this website, you agree to our , , and politics. We use at the center of preferences. © 2021 BioMed Central Ltd unless otherwise indicated. Part of .

Tongue Fasciculations - YouTube

Tongue Fasciculations Secondary to Hypoglossal Nerve ... | GrepMed

Chronic inflammatory demyelinating polyneuropathy with tongue fasciculation: A case report - ScienceDirect
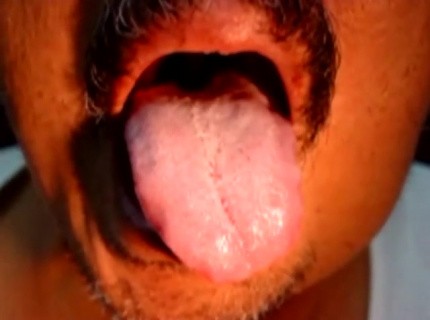
Fasciculations of A Tongue • Video • MEDtube.net
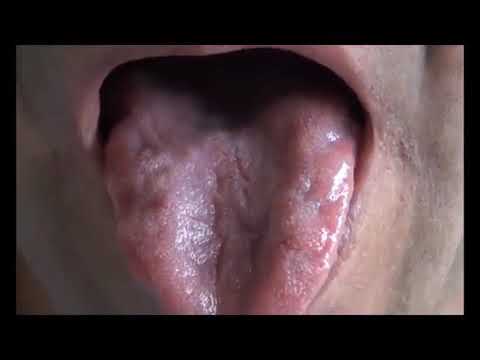
Tongue Fasciculations - YouTube

Atrophic tongue with fasciculation in patient 4 | Download Scientific Diagram

May 30 – Fasciculations | SunnyStrong

Teaching Video NeuroImages: Tongue fasciculations in spinal muscular atrophy - YouTube
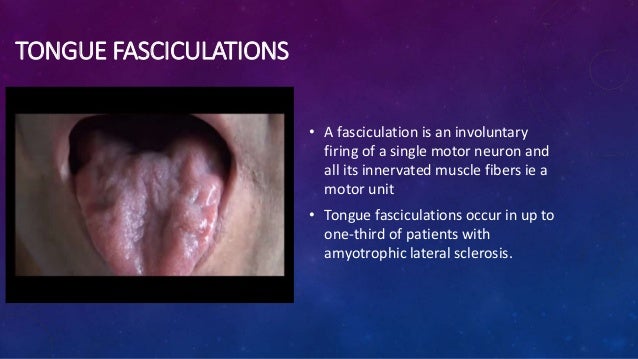
Disorders of the hypoglossal nerves

Unilateral Tongue Atrophy and Fasciculation | Cancer Biomarkers | JN Learning | AMA Ed Hub
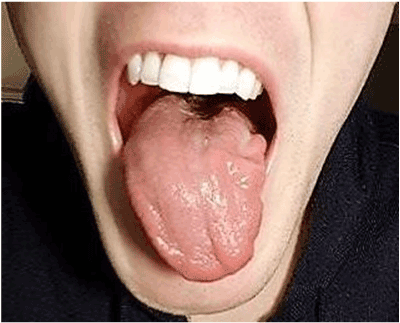
Persistent isolated unilateral hypoglossal nerve palsy

Tongue involvement in amyloidoses | Neurology
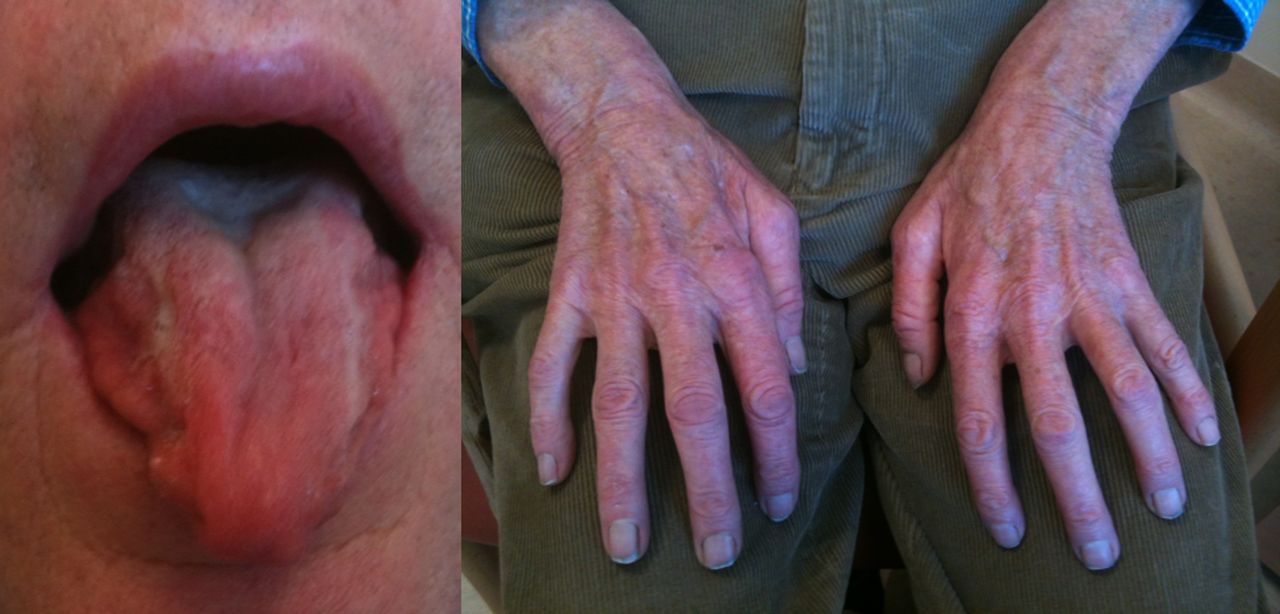
Mimics and chameleons in motor neurone disease | Practical Neurology
Tongue Fasciculations in the Newborn

Amyotrophic Lateral Sclerosis (ALS) - Neurology - Medbullets Step 2/3
FULL TEXT - Metastatic compressive lower motor neurone hypoglossal nerve palsy: A rare complication of prostate cancer - International Journal of Case Reports and Images (IJCRI)

Hettie vs SMA2 - Ever wondered what an SMA tongue 👅 looks...

Tongue fasciculations - YouTube

In this video, you see right sided atrophy, right sided ... | GrepMed
Tongue Myokymia and Earache as a unique presentation Associated with Local Tongue Pathology: A Case Report and Review of Literature - MDS Abstracts

Radiation‐induced tongue myokymia with hypoglossal nerve damage, mimicker of motor neuron disease - Memon - 2017 - Clinical Case Reports - Wiley Online Library
Persistent isolated unilateral hypoglossal nerve palsy
Neurology Pro - A DDx Tool on Twitter: "Tongue fasciculations… "

Patient with brain stem and spinal cord Lateral Sclerosis Amyotrophic
Tongue Fasiculations (SMA) on Vimeo

Tongue atrophy and fasciculations in transthyretin familial amyloid neuropathy | Neurology Genetics
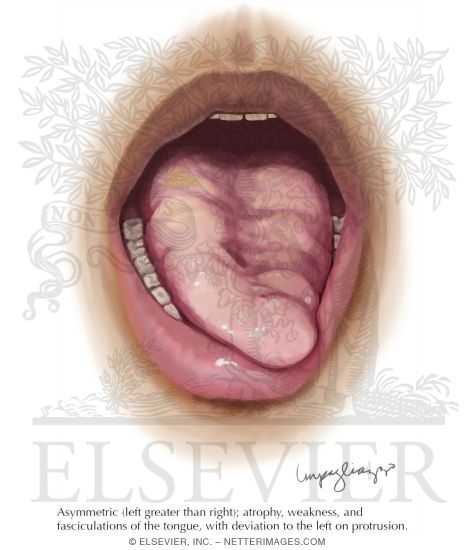
Tongue Atrophy In Amyotrophic Lateral Sclerosis
May 30 – Fasciculations | SunnyStrong
PDF) Tongue fasciculations with denervation pattern in osmotic demyelination syndrome: A case report of diagnostic dilemma

Bulbar Onset ALS vs. Normal Tongue Twitching » Scary Symptoms

Tongue Fasciculations in Organophosphate Poisoning | NEJM

Mimics and chameleons in motor neurone disease | Practical Neurology

Figure 2 from ALS: pitfalls in the diagnosis. | Semantic Scholar

Myokymia (fasciculation) of the tongue as a unique presentation of mucoepidermoid carcinoma - International Journal of Oral and Maxillofacial Surgery
Tongue Exam | Stanford Medicine 25 | Stanford Medicine
101111 als

Deviation of the tongue to the left and fasciculation. | Download Scientific Diagram
Tongue Fasciculations in Amyotrophic Lateral Sclerosis
Tongue Myokymia and Earache as a unique presentation Associated with Local Tongue Pathology: A Case Report and Review of Literature - MDS Abstracts
Fasciculation - Drone Fest
Posting Komentar untuk "fasciculations of the tongue"